DESCRIPTION OF WORKING GROUPS
The Action is organised in six multidisciplinary Working Groups.
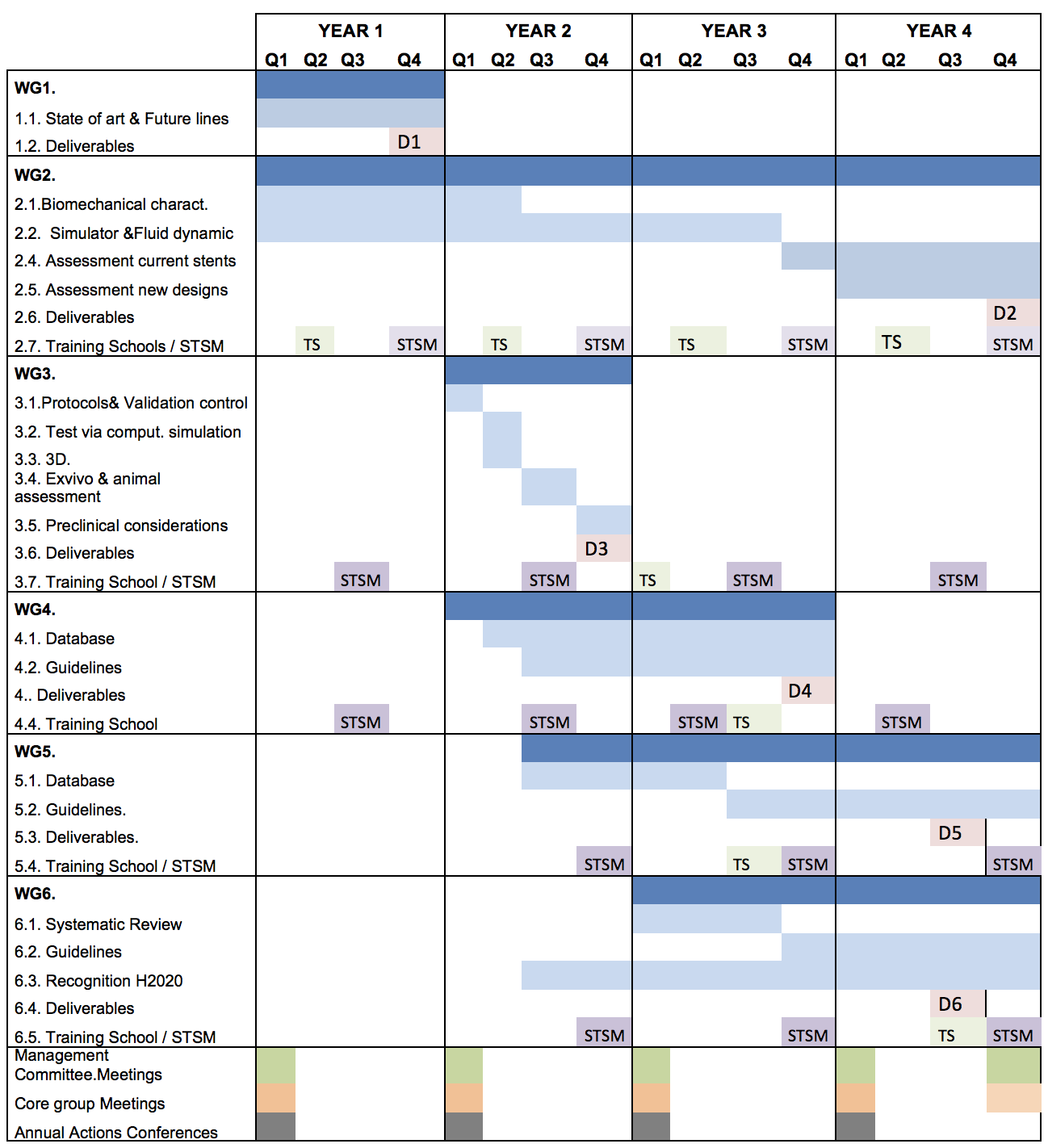
WG1. State of art of Urinary stents. This WG will focus its work in analysing the current literature on ureteral, urethral and prostatic stents from a multidisciplinary point of view. (M1-M12). Nowadays, there are only partial evaluations of the morbidity and causes of stent failure and it is necessary to know all the different approaches of the problem (urologic, industrial design, biomaterials in the urinary environment, inadequate therapeutic indications, etc.). In this WG1, involved experts are mainly urologists, but input from bioengineers can be valuable, as they could offer points of view that are not currently available.
Objectives: The objectives of the WG1 correspond to objective 1 listed in Specific objectives.
Tasks and activities. Members from this WG will be distributed according the type of stent to be analysed (ureteral, urethral and prostatic), to perform the literature review, conclude which are the causes of the complications and adverse effects and propose solution to these problems. The tasks and activities will summarize the state of art on-going research on this field, including possible lines of improvement after the assessment of the causes: Task 1. Ureteral stents, literature review; Task 2. Urethral stents, literature review; Task 3. Prostatic stents, literature review.
Milestones. Update urinary stent causes of failure (M6). Strategies for improvement (M12).
Deliverables. D1. Report on the state of the art in the field of urethral & ureteral stents, including scientific publications related to the WG1 of the Action (M12).
WG2. Computational simulation, Biomedical fluid dynamics, Biomechanical characterization. (M1-M44). The WG2 focuses its work in developing a computational setting to assess future stent designs. This is a world novelty, as nowadays there is no computational setting of this kind. In this WG2, engineers (in silico, fluid dynamics and mechanical characterization of the urinary system), are the main participants, with the support of expert urologists.
Objectives. The objectives of the WG2 correspond to the objective 4 listed in Specific objectives.
Task and activities in WG2 are distributed in: Task 1. Assessment-determination of the biomechanical characterization of the urinary tract.Task 2. The development of a realistic simulator of the urinary tract. Task 3. Application of more physical characteristics via computational fluid dynamics to the realistic simulator (fluid-structure interaction between urine flow and urinary tract’s wall).Task 4. Assessment of the current urinary stents, using the computational model of the urinary tract in order to validate their usefulness.Task 5. Evaluation, in the computational model, of the new designs proposed by the WG3.
Milestones. Database is created with all the specifications of the urinary tract and the biomechanical characterization of the components of the urinary tract, as well as the main materials used in the process of manufacturing stents (M14). The engineers create the computational environment of the urinary tract (M22). The completion of the in silico environment with the variables of fluid dynamics (M28). This computational setting is validated through the analysis of classic stents (M44).
Deliverables. D2. Report of the state of art of “In silico and urinary stents, biomechanical specifications in urinary tract, and computational simulation in urinary stents and future”, including scientific publications and yearly Training schools, meetings, workshops and STSM (M44).
WG3. Methodology for the development and validation of new stent designs.(M12-M24). The WG3 will be in charge of developing the Methodology and validation protocols of future urinary tract stents. Currently there are no guidelines describing the completion of the different stages of development, assessment and validation of a new design of urinary stent.
Objectives. The objectives of the WG3 correspond to the objective 2 listed in Specific Objectives.
Tasks and activities. The tasks to be performed are creating a methodology and validation protocols so can be used as general methodology in the development of new designs of urinary stents. This will facilitate future research groups the approach to this scientific and technical field.
Task 1. Protocol of objectives and improvements from the proposed design. Task 2. The production of a validation protocol (design, control, guidance for medical devices), with a Quality Management System and GLP considerations. Task 3. Tests via computational simulation.Task 4. The manufacturing methods using three-dimensional (3D) printing. Task 5. Exvivo assessment. Task 6. Animal model evaluation. Task 7. Pre-clinical testing considerations.
Milestones. 1. The production of a validation protocol (design, control, guidance for medical devices) (M16). 2. Tests via in-silico simulation, three-dimensional (3D) printing, Exvivo-In vivo assessment and Pre-clinical testing considerations (M24).
Deliverables. D3. Report on Comprehensive Methodology and Validation protocol on new stent designs, including scientific publications related to the WG3 of the Action (M24), with training school and STSM related to the WG3 of the Action (M24).
WG4. Biomaterials and stent coatings (M12-M36). The WG4 will work on the search of new biomaterials-nanomaterials and coatings with improved behaviour at urinary tract level when used for manufacturing stents. The aim with this WG is to determine the characteristics of the new biomaterials and coatings (polymeric, non-polymeric, biodegradables and/or metallic) that are involved in the improvement of stent success rate and stent-related morbidity.
Objectives. The objectives of the WG4 correspond to objective 3 listed in Specific objectives.
Tasks and activities. The tasks of this group are: Task 1. The creation of a Database of all the studied materials from the urologic stents field and also from other stent-related scientific fields (mostly the cardiovascular field). Task 2. The creation of a detailed and critic study of the characteristics of the biomaterials and coatings must meet to improve the response in the urinary tract, regarding both the interaction material-urinary environment.
Milestones. 1. Database of materials used in urinary stents and of those that could potentially be used in stents (M30). 2. Creation of Guidelines concerning the requirements for biomaterials and coatings exposed to the urinary environment (M36).
Deliverables. D4. Report on the State of art on new Biomaterials and Coatings suggest to Urinary environment, including scientific publications related with WG4 of the Action to the urinary environment (M36). During the third year, a Training School focused on the issues addressed by this WG4.
WG5. Drug Eluting Stents (DESs) (M20-M48). Following the idea of incorporating drugs onto the stent surface, several substances have already been used in DESs in an attempt to diminish stent-related adverse effects. An alternative novel tool for the confrontation of urothelial hyperplasia or Urinary tract infection following metallic stenting is expected to be provided from the use of DESs. Currently, this area of stent development is not being applied to urological practice; it must therefore be explored to learn if it can be of use, as it is nowadays with the vascular system. Creating a specific WG for the development of these stents is of great importance for the future of urinary stents. It is expected that with the improvement of the designs, Nano-Technology, making them more biocompatible, their features will be optimized and reduce side effects. The use of DESs in the ureter and urethra has been limited to few experimental studies. It remains to be proven if the promising benefit of DESs will eventually become a part of urological practice.
Objectives. The objectives of the WG5 correspond to objective 3 listed in Specific objectives.
Tasks and activities. Task 1. To create a Database of all the drugs assessed for DESs, considering stents and also other stent-related scientific fields (mostly vascular stents). Task 2. Creation of a detailed and critic study of the characteristics (Guidelines) required for these drugs to reduce the stents morbidity. Task 3. Suggestion of new research lines for future studies.
Milestones. 1. Database of drugs used in urinary stents (M30). 2. Creation of Report to agree the specifications drugs must accomplish to be released by urinary stents (M44).
Deliverables. D5. Report on Drugs assessed for DESs to reduce the urinary stents´s morbidity, including scientific papers and training school, meetings, workshops and STSM related to the WG5 of the Action (M44).
WG6. Future research lines (Bioactive-Antibody, Biocovered stents, Biodegradable, Nanotechnology, Bioprinting) (M24-M48). Coating of the stent surface is a developing field with significant potential for the reduction of stent-related complications. This WG is going to study, evaluate and suggest future lines of work that to this day, have not been raised for the development of urinary stents. Within the research lines that are going to be assessed there is an emphasis on: Bioactive Stents, Biomimetic or Biocovered Stents Nano-Technology applications and Biodegradable Stents. This WG will be leaded by experts in biomaterials, nano-materials, stent designs, cell therapy and coatings, along with the counselling of urologists.
Objectives. The objectives of the WG6 correspond to objective 5 listed in Specific objectives.
Tasks and activities. Task 1. To explore the potential of new technological platforms that may offer the opportunity to produce viable stents with the following features: bioactive properties, anti-body coated, biomimetic coatings, protein-interacting biomaterials, and biodegradable components or cell-coated (tissue engineering). Thus, a systematic Review will be performed about the advances in this field to assess its translation into the urology. Task 2. Presentation of the lines that are more likely to be successful in urological application.
Milestones.1. Systematic Review of the different technologic platforms suitable for stent development (M30). 2. Report, about the most appropriate technologic platforms to development of urinary stents (M48). Recognition of appropriate H2020 calls.
Deliverables. D6. Report on New technological opportunities in urinary stents manufacturing. Future in urinary stents, including scientific papers and training school, meetings, workshops and STSM related to the WG6 of the Action (M48).
GANTT DIAGRAM
PERT CHART (OPTIONAL)
RISK AND CONTINGENCY PLANS
This COST Action has many favourable conditions for the achievement of the proposed goals, as well as for the WG to develop their work appropriately. In the first place, the partners have great experience and knowledge. Due to the openness and inclusiveness of the network, it is foreseeable that the inclusion of the new groups will strengthen this network, preferentially in ITC or NNC countries. We can identify in COST Action the following risks:
- Risk. Distribution of the WGs’ topics allows the tasks to be developed independently by each WG in order to avoid deliverables delays. WG1 could be the only WG that might interfere. As a contingency plan, activities (MC, WGL, WGLV and SC) have been organized in a way that ensures a close communication between the different stakeholders of each WG. In order to be prepared for contingencies, everything will be coordinated through an Agenda detailing the WG work, the concrete distribution of tasks and deadlines for each of them. This Agenda will be provided an alarm-soft-reminder system to periodically notify the deadlines to the WGL-WGLV with anticipation.
- Risk. Unsatisfactory scientific results. Some WG (i.e. WG2) depends on their own scientific results and has low risk in some scientific results (computational models, in silico studies among others). As a contingency plan, it has been established that if the results from those tasks are not satisfactory, tests will be performed through an alternative method (physical simulation, ex vivo models, etc.).
- Risk. Low interaction between participants (and between WGs). Due to the high participation of the stakeholders it is necessary to assure the correct interaction and to avoid the work being monopolized by a single discipline or stakeholder. The WGL-WGVL elected represents all different areas of knowledge and the distribution of the WG members will include partners of all disciplines. Besides, the researchers selected for the control of the different WG has a wide knowledge of neighbouring disciplines. As a contingency plan, the MC-WGL-WGVL will supervise quarterly the presence of an active and interdisciplinary participation within the WGs.
- Risk. Low impact of Dissemination activities. Partners have wide experience in scientific publications, and in the organization of Scientific meetings. Nevertheless, as there are different disciplines collaborating together, a contingency plan has been described to the coordination: STSM coordinator inform to WGL-WGVL quarterly about any deviation for its amendment. Furthermore, owing to the great importance and participation in this Action of the STSM and training schools to pursue objectives 1-3-5-7, a permanent Contingency Committee has been arranged composed by STSM coordinator and AC. The mission of this committee is the continuous search of young researchers (ECI), preferably in ITC or NNC countries, and the evaluation of the ECI comments after STSM. An Agenda will be created for the Control of these activities, where all of them will be reflected, as well as the personal information of the activity Coordinator (AC, WGL, and STSM coordinator).
- Risk. Stakeholders’ communication. One of the main potential risks of this multidisciplinary network might be the failure in coordinating communications among stakeholders. To avoid this risk, the webpage of this COST Action will inform about all the activities and up-to-date deadlines for the meetings (Committee meetings, and annual conferences). It is a way to make sure that this Action count, in every WG, with the presence of different stakeholders. Also, with these measures, IRC, Patient Associations, Scientific Societies, Industrial Partners and Policy Makers will be controlled in a database to send all the notifications in order to assure their participation.
- Risk. Gender balance. Because of the risk detected among partners, the figure of GBC has been created. The role of GBC is to develop actions to enhance the participation of the under-represented gender, preferably in ITC or NNC countries. Finally, future H2020 projects (WG7) will depend on appropriate calls, a contingency that this Action cannot control.


